What are the different types of chemical bonds?
Heat us the degree of hotness or coldness of a body.The human body produces heat through a combination of processes, including: Metabolism The body metabolizes food to produce heat through chemical reactions and cellular respiration. The liver is a major source of metabolic heat. Muscle contractionsRead more
Heat us the degree of hotness or coldness of a body.The human body produces heat through a combination of processes, including:
Metabolism
The body metabolizes food to produce heat through chemical reactions and cellular respiration. The liver is a major source of metabolic heat.
Muscle contractions
Involuntary muscle contractions, like shivering, increase muscle cell activity and create heat. Voluntary muscle exertion and motion also produce heat.
Cellular respiration
Cellular respiration produces energy in the form of ATP, which is used for daily activities and the excess is released as heat.
Thyroid hormones
The hypothalamus releases thyroid hormones, which increase metabolic rate and heat production.
Blood friction
The friction of blood against blood vessel walls produces heat.
The body’s temperature is usually between 98 and 110 degrees Fahrenheit. The body’s ability to generate heat helps it resist exposure to low temperatures.

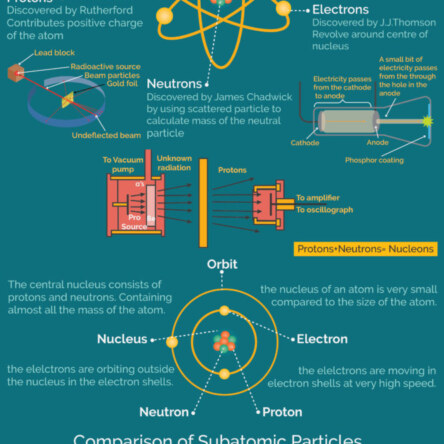







A chemical bond is a force of attraction between atoms or ions. Bonds form when atoms share or transfer valence electrons. Valence electrons are the electrons in the outer energy level of an atom that may be involved in chemical interactions.The four major types of chemical bonds are: Ionic bonds FoRead more
A chemical bond is a force of attraction between atoms or ions. Bonds form when atoms share or transfer valence electrons. Valence electrons are the electrons in the outer energy level of an atom that may be involved in chemical interactions.The four major types of chemical bonds are:
See lessIonic bonds
Form when one atom transfers electrons to another, creating oppositely charged ions. Ionic bonds are important for many processes in chemistry, including the development of batteries and the production of glass.
Covalent bonds
Form when atoms share electrons, creating electron pairs that surround the nuclei of the atoms. Covalent bonds are common in organic compounds, which contain carbon.
Metallic bonds
Form when electrons are shared between multiple metal atoms, creating a “sea of electrons” that orbits the nuclei of the atoms. Metallic bonds are strong, which explains why metals have high melting and boiling points, and are good conductors of heat and electricity.
Hydrogen bonds
Form when a hydrogen atom covalently bonded to an electronegative atom interacts with another electronegative atom. Hydrogen bonds are weak electrostatic bonds.
In reality, most materials have more than one type of bonding. For example, iron has mostly metallic bonding, but also some covalent bonding.